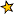No, there is no limit - there is an infinite number of energy levels ("electron shells") in an atom.
Consider the simplest atom, hydrogen. Its single electron will be at energy level 1, nearest the nucleus, if the atom has minimum energy i.e. in the ground state.
By absorbing UV light of the correct wavelength the electron can gain sufficient energy to be promoted to energy level 2. Again, by absorbing visible light of the correct wavelength the electron can then be promoted to level 3. Similarly, by absorbing IR light of the correct wavelength the electron can be further promoted to level 4. These electron shifts produce absorption spectra.
This process can be repeated until the atom has absorbed sufficient energy that the electron reaches the infinite energy level i.e. it has broken free from the attraction of the nucleus and is independent. This is the ionisation energy of hydrogen, 1312 kJ/mol.
Similarly for atoms of all other elements.
 https://opentextbc.ca/universityphysicsv3openstax/chapter/bohrs-model-of-the-hydrogen-atom/
https://opentextbc.ca/universityphysicsv3openstax/chapter/bohrs-model-of-the-hydrogen-atom/
The Bohr model of the atom provides a theoretical basis for explaining the line spectra of hydrogen atoms. Based on a planetary model of the atom, Bohr hypothesized that an electron could only exist in quantized energy levels, with the electron orbiting the nucleus at a fixed radius. The allowed quantized energy levels depend on the value of an integer n, called the principal quantum number, which can take any value in the range 1,2,3, .... infinity.