Question #144424. Asked by happy1234512.
Last updated Jul 24 2017.
Originally posted Jul 23 2017 10:39 PM.
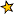

The ultraviolet catastrophe, also called the Rayleigh-Jeans catastrophe, was the prediction of late 19th century/early 20th century classical physics that an ideal black body at thermal equilibrium will emit radiation in all frequency ranges, emitting more energy as the frequency increases. By calculating the total amount of radiated energy (i.e., the sum of emissions in all frequency ranges), it can be shown that a blackbody would release an infinite amount of energy, contradicting the principles of conservation of energy and indicating that a new model for the behaviour of blackbodies was needed.(quoted from McQuarrie, Donald A.; Simon, John D. (1997). Physical chemistry: a molecular approach (rev. ed.). Sausalito, Calif.: Univ. Science Books. ISBN 978-0-935702-99-6 in the following Wikipedia article)
The term "ultraviolet catastrophe" was first used in 1911 by Paul Ehrenfest, but the concept originated with the 1900 derivation of the Rayleigh-Jeans law. The phrase refers to the fact that the Rayleigh-Jeans law accurately predicts experimental results at radiative frequencies below 105 GHz, but begins to diverge with empirical observations as these frequencies reach the ultraviolet region of the electromagnetic spectrum.
|
|
|
|